Queen’s University Belfast create new groundbreaking £12M research centre
This £12M Centre will focus on improving the efficiency of rare disease trials and increasing the number of opportunities for patients to take part using a UK ‘4 nations’ approach to deliver trials of new treatments.
-1600x1066.png)
- Globally, there are more than 450 million people living with rare diseases.
- For most people living with rare disease there is no cure.
- 95% of rare diseases do not have an approved therapy.
Clinical trials for rare diseases are challenging and can be a major limiting step in getting new treatments to patients. The research can be fragmented, and researchers sometimes lack access to specialist facilities, as well as advice on maximising trial recruitment and keeping people within trials, managing data and trial regulations, optimising trial designs, and translational project management. Our new LifeArc Centre for Acceleration of Rare Disease Trials brings together a consortium of three universities from across the UK to boost the capacity and efficiency of rare disease trials across the UK. Queen’s University Belfast, Newcastle University, and University of Birmingham are pooling their expertise in a partnership with a 4-nation approach. This £12M Centre will focus on helping patients have access to appropriate treatments earlier, delivering trials of new treatments using ‘one stop’, patient friendly, models and facilitating equitable recruitment. We will speed up the delivery of clinical trials for people with rare diseases and enable more rapid approval of new therapies for use in the NHS.
Are people affected by rare disease working on the project?
Absolutely, our rare disease community raised many important barriers to new treatments with differences very clear across the UK and with Europe. We are working with people living with rare disease(s) and their families to ensure 'nothing about us without us' trial designs and delivery, ensuring the right information is collected that improves patient and / or family life, and enabling treatments to be approved more quickly. People affected by rare disease are involved at every stage in this project from the initial idea, designing the project, as formal investigators on the funding application, leading individual elements, and helping deliver the LifeArc Centre for the Acceleration of Rare Disease Trials. We have a broad outreach and are keen to welcome participation and involvement throughout.
What does this mean for Northern Ireland?
A lot! This is a very exciting initiative with lots of potential. We know that Northern Ireland is underrepresented in clinical trials, in clinical research with children, for rare disease research funding, and for rare disease clinical centres of expertise. This project, and other complementary research, will help improve research opportunities and facilitate access to treatment for people living with rare diseases.
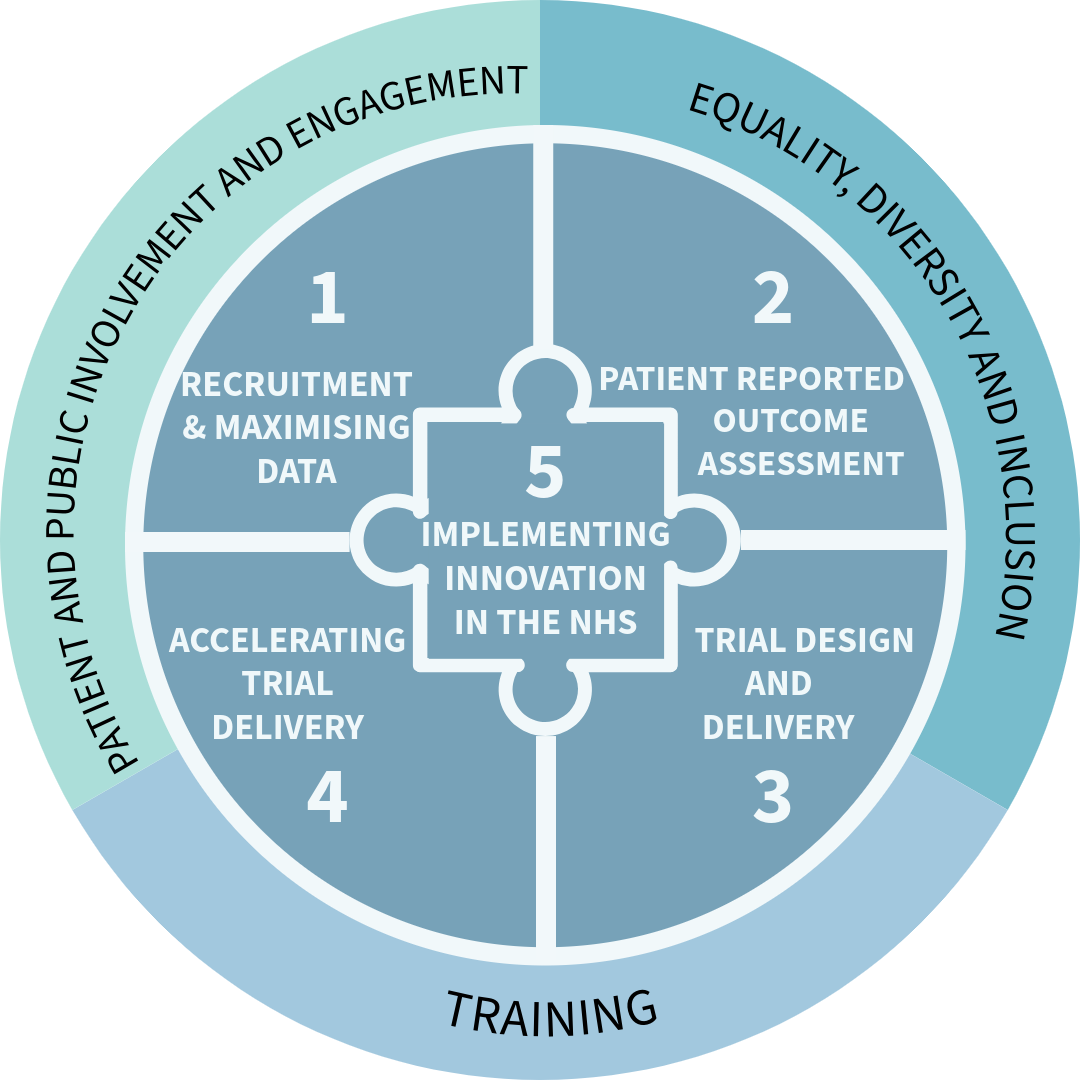
Work package 1:
- We are developing a consent register for Northern Ireland where people can sign up to be kept informed about various research opportunities.
- We are developing a secure online recruitment portal where people living with rare diseases can self-register their interest in clinical trials, linked to a clinical trial finder. Effectively, this will help link patients with clinical trials for which they may be eligible.
- We are developing core datasets, standardised data dictionaries that improve coding of rare diseases in electronic care records, and e-cohorts to support pre-screening of patients, collect 'real world' natural history data to inform patient reported outcomes, and enabling the use of innovative trial designs, patient simulation with digital twins, synthetic controls for innovative trial designs, and more personalised medicine. NI has an exceptional opportunity to lead the way using national population-based datasets with validated, quality controlled information for rare diseases.
Work package 2:
- We will develop a standardised approach to patient reported outcomes for rare disease trials, including developing consensus guidelines that support equity for trials
- We will identify what prevents inclusive collection of patient reports outcomes and explore how better approaches can be used / developed
- We will develop a bespoke design service for patient reported outcomes
Work package 3:
- Focuses on creating rare disease clinical trial capacity to support delivery of a portfolio of trials, integrating innovative trial designs, and delivering trials at scale. Newcastle and Birmingham have more impact in this WP as they already have established rare disease centres of excellence and rare disease trial units...Belfast is scaling up!
Work package 4:
- Will set up a rare disease trials network across Scotland, England, Wales, and Northern Ireland.
- We will work with NHS colleagues to develop a system so that one approval can be supported by multiple recruiting centres.
- In NI we will also set up an active rare disease clinical-academic research network, informing and enabling clinical trials
- We are putting in place core staff to help get local patients involved in clinical trials locally and / or overseas. A patient logistics and experience officer will support individual patients and their families from initial consent, through their trial journey, to completion of the trial.
- We are also working to de-centralise trials - enabling home health monitoring and / or recruitment supported by GPs
Work package 5:
- Supports implementation of rare disease trial innovations in the NHS, developing new approaches and learning from international experts what works, when.
- We will conduct health economic analyses, constructing a Value of Information (VoI) framework and developing a standardised approach to patient-informed risk benefit trade-offs through Multiple Criteria Decision Analysis (MCDA)
- We will identify barriers to participation in clinical trials for our rare disease community, working with stakeholders to identify solutions
- We will identify regulatory barrier to accessing / sharing data and for approvals / access to treatment across the UK
- A Citizen's jury will address patient-relevant issues around early access to medicines
- We will work with the UK RD_IMAG group and international collaborators to maximise benefits for our rare disease community.
There are dedicated workpackages for patient involvement and engagement, as well as for equity, diversity and inclusion, ensuring a collaborative and equal contribution across all research. We are also helping build capacity for rare diseases across Northern Ireland, with a dedicated rare disease doctoral training program that will train the next generation of laboratory, computational, and clinical, researchers.
Professor Amy Jayne McKnight from the School of Medicine, Dentistry and Biomedical Sciences at Queen’s, who is co-lead in the project said: “Across Northern Ireland (NI), there are more than 110,000 people living with rare conditions. That is one in every 17 people in our population. Less than 5% of rare diseases have an approved therapy, so more trials with innovative designs are urgently required. I’m looking forward to sharing expertise with colleagues including Professor Dave Jones and Volker Straub, Newcastle University and Professor Tim Barrett, University of Birmingham, as we establish and develop this crucial rare disease initiative.
“For NI, we are also supported by Health and Social Care Research and Development. This new Centre will establish a collaborative NI rare disease trials hub that will support in building local infrastructure and skills and allow capacity for trial design and delivery. In addition, the Centre will aid in the development of practical tools to support patients and doctors in participating in research, creating opportunities for international research and further investment, whilst ensuring the voices of patients and families remain at the centre of this progress. Lived experience from patients and their families across NI was crucial in helping shape the design of this Centre to be of maximum benefit.”
Kerry Leeson-Beevers is the parent of a child with the rare genetic condition, Alström Syndrome, which often causes loss of vision and hearing, and can lead to serious life-threatening problems with the heart, liver and kidneys. Kerry, who is also CEO of Alström Syndrome UK, said: “We have no specific treatment for Alström Syndrome and when my son, Kion, was a baby, I was told it could take around 10 years for any treatment to be developed. 20 years later, we are still waiting. People living with rare conditions don’t have the luxury of time and the mainstream way of delivering healthcare and drug development rarely works for people with rare conditions. As a mum and the Chief Executive of Alström Syndrome UK, having a centre that will deliver a coordinated, inclusive and supportive approach to accelerate clinical trials gives me great hope.”
Professor Ian Young, Chief Scientific Advisor to the Department of Health and Director of Research for Health and Social Care, said: “As Chair of the Northern Ireland Rare Diseases Implementation Group (NIRDIG), I very much welcome this announcement which will have enormous benefit for people, and their families, living with a rare condition in NI.
“Improving access to specialist care, diagnosis and treatments, and increasing participation in rare disease research are key priorities for the NI Rare Diseases Action Plan. Today’s announcement will drive forward those actions, harnessing rare disease expertise from across the UK to improve design of translational rare disease research studies, facilitate delivery of clinical trials, build much needed local capacity, and improve access to innovative and specialist rare disease treatments for our patients. I would like to thank Professor McKnight and her colleagues, for their leadership, hard work and commitment in making this exciting opportunity a reality for NI.”
Dr Patricia O’Hare, NI Paediatric Clinical Lead for rare diseases said: “I am delighted with the announcement of this successful funding for rare disease trials in NI. As a paediatrician involved in clinical trials, I have seen how research can change our ability to treat rare diseases in real time. Bringing effective treatments to children and adults with a rare disease or condition, in addition to opportunities to participate in research, is at the core of what we are working towards in NI. The LifeArc centre for acceleration of rare disease trials will demonstrate the benefit of collaborative research and we are excited to be involved in this innovative centre.”
Dr Catriona Crombie, Head of Rare Disease at LifeArc, says: “We’re extremely proud to be launching four new LifeArc Translational Centres for Rare Diseases. Each centre has been awarded funding because it holds real promise for delivering change for people living with rare diseases. These centres also have the potential to create a blueprint for accelerating improvements across other disease areas, including common diseases.”
The LifeArc Centre for Acceleration of Rare Disease Trials, along with the the LifeArc Centre for Rare Respiratory Diseases, LifeArc Centre for Rare Kidney Diseases, and LifeArc Centre for Rare Mitochondrial Diseases, has been awarded a share of nearly £40M over five years from the not-for-profit medical research charity, LifeArc. Each centre will tackle an area of unmet need, to unlock science, accelerate medical progress, and have the greatest impact for patients.

Media
Please do contact our rare disease team by email for further information.

